Dr Fusco's High Fiber Diet

Article Menu
/ajax/scifeed/subscribe
Open Access Review
Inflammatory Bowel Diseases: Is There a Role for Nutritional Suggestions?
1
Department of Translational Research and New Technologies in Medicine and Surgery, University of Pisa, 56100 Pisa, Italy
2
Department of Medical Sciences, University of Turin, 10126 Turin, Italy
3
IBD Unit, Department of General Surgery and Gastroenterology, Pisa University Hospital, 56124 Pisa, Italy
*
Author to whom correspondence should be addressed.
†
These authors contributed equally to this work.
Academic Editor: Maria Cappello
Received: 1 April 2021 / Revised: 17 April 2021 / Accepted: 18 April 2021 / Published: 20 April 2021
Abstract
Nutrition has an important impact on inflammatory bowel diseases (IBD). In particular, several studies have addressed its role in their pathogenesis, showing how the incidence of IBD significantly increased in recent years. Meanwhile, nutrition should be considered a component of the treatment of the disease, both as a therapy itself, and especially in the perspective of correcting the various nutritional deficiencies shown by these patients. In this perspective, nutritional suggestions are very important even in the most severe forms of IBD, requiring hospitalization or surgical treatment. Although current knowledge about nutrition in IBD is increasing over time, nutritional suggestions are often underestimated by clinicians. This narrative review is an update summary of current knowledge on nutritional suggestions in IBD, in order to address the impact of nutrition on pathogenesis, micro- and macro-nutrients deficiencies (especially in the case of sarcopenia and obesity), as well as in hospitalized patients.
1. Introduction
Inflammatory bowel diseases (IBD) are chronic relapsing diseases of unknown origin affecting the gastrointestinal tract [1]. Diet is one of the key factors in their pathogenesis and outcome, since it could have a pro-inflammatory effect [2]. Notably, the incidence of IBD worldwide has significantly increased in the last decades [3,4], and it could be related to differences in lifestyle, including the adoption of a western diet, characterized by high amounts of proteins and saturated fats, in concomitance with low amounts of vegetables, fibers, and fruits [5].
Patients with IBD are particularly aware of the dietary influence on their disease, since approximately 70% of them think that diet could influence their condition [6], 60% consider diet to play a major role in inducing a relapse [7], and 16% are convinced that diet could initiate the disease [8]. Interestingly, in order to modify their dietary habits, patients frequently tend to avoid certain foods rather than increasing the intake of dietary components with presumably more beneficial properties [9].
Several dietary factors have been suggested to have a potential causative role in IBD [10]. On the other hand, diet components may have therapeutic implications, being able to correct nutritional deficiencies as well as to exert anti-inflammatory properties [11].
However, although in recent years, clinical and experimental research significantly increased the therapeutic armamentarium [12], few data are currently available for dietary suggestions. Some guidelines included specific formula diets as induction or adjunctive therapy for Crohn's disease (CD), while there is no specific recommendation for ulcerative colitis (UC) [13]. This narrative review aims to summarize current knowledge on nutritional suggestions in IBD, with the perspective to address the impact of nutrition on pathogenesis, micro- and macro-nutrients deficiencies (especially in the case of sarcopenia and obesity), as well as in hospitalized patients.
2. Nutrition in IBD Pathogenesis
IBD are multifactorial diseases in which genetic predisposition, altered immune system, dysbiosis, and environmental factors contribute to disease onset and recurrence [1].
In the last decades, the incidence of IBD in developing countries, but not in the Western world, increased significantly: Since genetic changes manifest over large time frames, external factors have been called into question [4]. Migrants from low- to high-prevalence regions tend to acquire a "high-prevalence" pattern of IBD onset in two generations [14]. The adoption of a low-fiber diet, rich in saturated fats, refined sugars, processed foods, associated with a diet-induced gut dysbiotic profile, appears to play a major role in the link between environment and inflammation [5,15].
2.1. Fat and IBD
2.1.1. Saturated Fats
Saturated fats are found mainly in animal products, such as meat and dairy products.
In-vivo studies in animal models found that a diet rich in high saturated fats promotes chronic inflammation [16], although the mechanism is still unknown. One explanation is that the amino acid taurine, present in saturated fats, linked to bile acids, seems to increase substrate availability for sulfur-reducing bacteria like Bilophila Wadsworthia, highly prevalent in the dysbiotic microbiota of IBD patients. Furthermore, Muhomah et al. [17] found that saturated fats are able to reduce the level of secretory immunoglobulin (sIg) A, altering the immune response to intestinal microbiota.
In addition to the risk of developing IBD, a diet rich in saturated fatty acids seems to increase the relapse risk, particularly in UC [18].
2.1.2. Polyunsaturated Fats (Omega-3)
Omega-3 fats, contained in olive oil, are powerful antioxidants and are associated with a lower risk of IBD, both in UC [19,20], as well as CD: Children that consume a diet rich in omega-3 fats are at lower risk of CD development [21].
2.2. Red Meat and IBD
The consumption of red meat, due to its high content of saturated fat and cooking method, has been linked to an higher risk of colon cancer and inflammation [22]. Red meat is metabolized by intestinal bacteria with production of branched-chain amino acids and toxic elements like hydrogen sulfide, nitrous compounds, amines, and ammonia that induce DNA damage of eucaryotic cells and promote colon inflammation in murine models [23,24]. Notably, large epidemiological data confirmed the association between high consumption of red meat and risk of IBD development, in particular UC [25], where it was found to affect also the relapse risk [26].
2.3. Sugars and IBD
Sugars are part of everyone's diet. The modern diets are rich in sugar; chocolate, cookies, cakes, ice creams, fruit punches, energy drinks, soft drinks, iced tea, and lemonade contribute to the "pandemic" of obesity, diabetes, and steatosis that plagues the western world [27]. Sucrose, a disaccharide composed of monosaccharides fructose and glucose, is the most common sugar found in processed foods. Many studies have linked high sugar intake with IBD incidence [28,29,30,31].
The pathogenic mechanism that links high sugar intake with the onset of IBD is not fully elucidated. A possible explanation is a reduction of intestinal mucus [32]. A sugar-rich diet favors the increase of Akkermansia muciniphila, a mucolytic bacterium. The mucus layer separates luminal bacteria from intestinal epithelium: A thinner mucus layer allows bacteria to come in contact with the epithelial cells, eliciting an inflammatory response. In addition, this type of diet increases the percentage of pro-inflammatory Sutterellaceae and Marinilabiliaceae, which induce bowel inflammation [33], and reduce bacteria with anti-inflammatory properties like Lachnospiraceae and Lactobacillaceae, able to produce the short-chain fatty acid (SCFA) butyrate, the main anergy source of enterocytes [34].
2.4. Fiber and IBD
In contrast to eastern and African diets, the western diet is low in fiber. Butyrate-producing bacteria, like those belonging to Bacteroidetes and Firmicutes phylum, exert their anti-inflammatory activity metabolizing dietary fibers: A low-fiber diet leads to reduced production of butyrate, which acts as a negative regulator of pro-inflammatory pathways and enhances the intestinal barrier function [35]; this, in turn, increased the risk of IBD onset [36]. When substrate is scarce, intestinal bacteria use the intestinal mucus as a nutrient, which leads to inflammation through close contact between bacteria and the epithelial layer [37]. Long-term intake of fibers from fruit has been shown to be protective against the development of CD, but not of UC [38,39,40,41,42].
The benefit of fibers in IBD remission is uncertain. Brotherton et al. [43] found that a low fiber consumption in CD during remission is associated with a higher risk of clinical relapse, but in the case of stricturing CD, the consumption of dietary fibers could precipitate obstruction.
2.5. Nuts and IBD
A diet rich in nuts and dried fruits, due to their high content of omega-3 fatty acids, fibers, and antioxidants, has been shown to decrease the cardiometabolic and inflammatory risk; the beneficial effects on health include reduced risk of cancer, diabetes, neurological diseases [44]. A diet rich in walnuts protects mice from dextran sulfate sodium (DSS)-induced colitis [45]. The mechanism of action seems to be a decrease of macrophages activation, reduction in the production of pro-inflammatory cytokines like IL-6, IL-8, IL-1α, tumor necrosis factor (TNF), and an increase of anti-inflammatory cytokine IL-10.
2.6. Vitamin D and IBD
The health benefits of vitamin D are pleiotropic and include bone health, modulation of the immune system, antimicrobial protection, and mucosal integrity [46]. Deficiency of vitamin D is common in patients affected by IBD, in particular CD, due to malabsorption, and is worsened by reduced sunlight exposure and steroid treatment [47].
The positive effects of vitamin D are related to the improvement of epithelial barrier function [48] T-cells development, production of anti-inflammatory cytokines, and modulation on both innate and adaptive immunity [49], but even to antimicrobial peptides secretion [50]. In in vivo experiments, inadequate dietary intake of vitamin D promotes colitis development [51]. One possible explanation for this observation is that vitamin D deficiency produces intestinal dysbiosis, with a reduction of bacteria with anti-inflammatory properties (e.g., Firmicutes) and an increase in pathobiontic bacteria (e.g., Bacteroides and Proteobacteria) [52]. Moreover, even clinical observations have frequently found a link between low levels of vitamin D and a more aggressive course of IBD [53,54,55].
2.7. Emulsifiers and IBD
The western diet contains large amounts of emulsifiers, widely used in processed food to improve food appearance, texture, and palatability due to their intrinsic properties. Emulsifiers affect the gut microbiota, disrupt the mucosal barrier, and promote inflammation; in mice models, they induce metabolic syndrome, colitis, and translocation of Escherichia coli [56,57].
3. Nutritional Suggestions in Outpatient Management
Prevalence of malnutrition in IBD patients has been reported between 20% and 85%, with the highest prevalence occurring in hospitalized CD patients [58,59] and include both macro and micronutrients; the nutritional assessment is a crucial part of clinical evaluation [60]. Lean mass loss or sarcopenia should be regarded as a separate aspect of malnutrition and independently treated, even in patients with a normal body mass index (BMI) [61].
Several screening tools have been developed for the diagnosis of malnutrition, which includes phenotypic (age, weight loss, BMI) and etiologic (disease activity, food intake) criteria. The most important screening tools are the Saskatchewan IBD Nutrition Risk Tool, Malnutrition Inflammatory Risk Tool, Malnutrition Screening Tool, Nutrition Risk Screening (NRS) tool, and the Malnutrition Universal Screening Tool (MUST) [62].
A multidisciplinary approach and close collaboration with expert dieticians are crucial for the correct management of these patients, particularly those affected by short bowel syndrome and intestinal failure.
About 15–40% of adults with IBD are obese, and an additional 20–40% are overweight, equally distributed between CD and UC [63]. Data suggests that obesity could promote rapid clearance of biologic drugs reducing the response to therapy and increases perioperative complications [63]. Obesity and sarcopenia in IBD patients can lead to sarcopenic obesity, which negatively impacts the patient's status, increasing disability, mobility [61,63].
A summary of the role of nutrients in inflammatory bowel disease is shown in Figure 1.
3.1. Micronutrients
In Table 1, the main micronutrients that deserve attention in patients affected by IBD are reported.
3.1.1. Iron
Iron deficiency is highly prevalent in patients affected by IBD, and about 20% of them suffer from iron deficiency anemia [70]. The main causes of iron deficiency are iron malabsorption in CD and loss from the gastrointestinal tract, especially in the case of active UC (whose hallmark symptom is bloody diarrhea). However, ulcers of colonic/ileal CD can lead to a less clinically evident, but not less severe, anemia [64]. A thorough assessment of iron metabolism parameters, including iron serum levels, transferrin (with transferrin saturation), and ferritin allows differentiating iron deficiency from anemia related to chronic diseases or combined anemia, which commonly occur. In iron deficiency anemia, low serum levels of iron, transferrin saturation, and ferritin are observed; conversely, in inflammatory anemia, ferroportin-1 is inhibited due to high hepcidin levels, and iron is sequestered in its deposits which leads to high ferritin and low transferrin serum levels [71]. In patients affected by CD with extensive small bowel inflammatory involvement or resection, iron malabsorption represents an additional causative factor. In combined anemia, ferritin levels may appear falsely normal, reflecting intermediate values between those induced by iron deficiency and inflammation, respectively [72]. However, even additional factors (renal failure, hemolysis) can contribute to worsening the anemia in IBD patients.
Iron is found in a lot of foods, including beef, liver, fish, poultry, eggs, legumes. Regarding the treatment of iron deficiency anemia, there is a heated debate regarding the best method of administration. Most authors favor intravenous (i.v.) administration due to a theoretical risk of IBD relapse due to oral iron administration [73], but recent publications have demonstrated the safety and efficacy of new oral iron formulations [74,75]. In our opinion, i.v. administration should be limited to patients with an iron deficiency anemia not responding or intolerant to oral iron administration.
3.1.2. Folate
Folate is a cofactor in DNA synthesis. Folate deficiency is the second most common micronutrient deficiency in patients with IBD, affecting about 30% of patients with CD and 10% of patients with UC [65]. The major causes are malabsorption (mainly in extensive CD with proximal small bowel malabsorption involvement or short bowel syndrome) or low intake, insufficient to counterbalance the blood loss from the gastrointestinal tract [76]. Further causes of folate deficiency are medications used to treat IBD like methotrexate or salazopyrine, which inhibit folate absorption [77]. In IBD patients with folate deficiency, celiac disease should be excluded by assessment of serum anti-tissue transglutaminase antibodies [78].
The main consequence of folate deficiency is megaloblastic anemia. Low folate levels can be associated with hyperhomocysteinemia, with an increased risk of thrombotic events [79].
Foods rich in folate are lentils, beans, vegetables, leafy greens, and citrus fruits. In addition to dietary advice, in the case of folate deficiency, 5 mg of folate per day is recommended with secondary use of methotrexate.
3.1.3. Vitamin B12 (Cyanocobalamin)
Approximately 20% of CD patients develop vitamin B12 deficiency [66], because vitamin B12 is selectively absorbed in the terminal ileum, the most commonly affected segment in CD, which sometimes is even resected [80]. Although mucosal inflammation and/or insufficient oral intake are the major cause of deficiency in these patients, in case of severe or not fully explained deficiency, autoimmune gastritis should be excluded by measuring anti-parietal cell antibodies.
Clinical manifestations of vitamin B12 deficiency include megaloblastic anemia and peripheral neuropathy [81].
Foods rich in vitamin B12 are animal products, including fish, tuna, shellfish, beef, liver, poultry, eggs, dairy products. Vitamin B12 deficiency is supplemented with 5000 µg of intramuscular cyanocobalamin once monthly, and more recently, sublingual formulations have been introduced [82].
3.1.4. Vitamin D
We treated the role of vitamin D in IBD pathogenesis and recurrence in the section dedicated to "Nutrition in IBD—Pathogenesis".
Vitamin D deficiency is highly prevalent in IBD, involving up to 90% of malnourished and roughly 80% of apparently well-nourished patients [7,67]; a negative association was described between vitamin D serum levels and BMI [83].
Dietary intake of foods rich in vitamin D, such as dairy products (milk, yogurt), eggs, liver, cod liver oil, salmon, provide only a minor proportion of daily needs. Skin exposure to solar ultraviolet B radiation is the major source of vitamin D; in young adults, a summer sun exposure of about 25% of body surface (face and arms) for 15 min twice or thrice a week is equivalent to oral intake of 25 µg (1000 IU) [84]. All patients treated with steroids must be supplemented with at least 25.000 IU of cholecalciferol per month orally; patients with serum 25-OH vitamin D lower than 20 ng/mL must be supplemented with at least 25.000 to 50.000 IU of cholecalciferol per month orally [67]. If levels are <10 ng/mL, 50.000 IU per week for 6–8 weeks, then 800 IU daily are recommended [85]. In case of severe malabsorption, injectable formulations may be advisable, and the dosage of 300.000 IU intramuscularly every six months has been suggested [86,87].
3.1.5. Zinc
Zinc is an essential trace element and plays an important role in wound repair, as well as being involved in the regulation of the immune system and in maintenance of balanced intestinal permeability [88]. Although the assessment of its serum levels is not the current practice of gastroenterologists, with the exception of patients with intestinal failure, it has been estimated that 15% of IBD patients suffer from zinc deficiency [69]. However, the reliability of the zinc measurement is questionable due to fluctuations with intake [68,89]. As low zinc levels have been associated with poor clinical outcomes in IBD, close monitoring and replacement of zinc in IBD patients should be suggested, especially in those with diarrhea [90].
Foods rich in zinc are meat (especially liver), seafood, eggs. Zinc supplementation requires 40–110 mg three times a day for 8 weeks orally and subsequent re-evaluation [91].
3.2. Macronutrients
Macronutrients include carbohydrates, proteins, and fats. In the section "Nutrition in IBD—Pathogenesis" we have already described how the western diet, rich in sugar, red meat, and saturated fats, and low in fiber, is considered pro-inflammatory [92] and could increase the risk of IBD. On the other hand, macronutrient deficiency may occur in patients with active IBD who reduce the oral food intake due to nausea/vomiting, abdominal pain, or fear of worsening symptoms during flares, as well as in case of stricturing CD. In case of severe macronutrient deficiency, such as in short bowel syndrome complicated by intestinal failure, a multidisciplinary approach with an expert dietician is mandatory and parenteral nutrition (PN) may be needed [93].
No specific dietary intervention has been demonstrated to promote remission in adult patients with active IBD [94]. The role of exclusive enteral nutrition (EEN), which in children with CD seems as effective as steroids in inducing remission [95], will be discussed in section "Nutritional therapy during hospitalization". However, enteral nutrition support based on a polymeric liquid formula rich in TGF-β (Modulen IBD®) has proved to be effective in pediatric IBD [96]. Several "anti-IBD" diets have been proposed (e.g., the Specific Carbohydrate Diet, the Paleolithic diet, the IGG4 exclusion diet, and Semivegetarian diet), but currently, their diffusion among the majority of gastroenterologists and the data supporting their efficacy are scarce [97].
3.3. Sarcopenia
The assessment of sarcopenia in patients with active IBD was recently introduced into clinical practice. Sarcopenia, traditionally related to aging [98], is defined as a decrease of lean (skeletal) muscle mass with consequent decreased muscular strength, and it is a consequence of decreased mobility, chronic inflammation, malnutrition [99]. Patients with reduced oral intake, severe inflammation, or short bowel syndrome in CD, especially in the elderly, are prone to catabolism of muscle tissue. Drugs used to treat IBD, in particular systemic steroids, contribute to decrease muscle mass due to stimulating myostatin [100]. Sarcopenia represents a predictive factor for surgery and increases the rate of postoperative complications [101,102]. A mere evaluation of BMI in patients affected by IBD is misleading: In fact, body composition could be altered, with a reduced lean mass compensated by an increase in fat mass [103]. So, tests like hand-grip strength are mandatory to look for the disease [104]. Recently, it has been recently shown that the ratio between thyroid hormones, expression of decreased lean muscle mass, could be used as an indirect therapeutic biomarker of response to biological therapy in elderly patients with IBD [105]. Indeed, patients with an oral caloric intake insufficient to cover the metabolic needs (short bowel syndrome in CD, reduced oral intake due to obstructive symptoms, or severe inflammation), especially in the elderly, are prone to catabolism of muscle tissue. Physical activity is the most efficient way to increase muscle mass [106].
3.4. Obesity and IBD
The obesity "pandemic" of western countries do not spare patients affected by IBD: About 25% of these patients are obese and about 30% are overweight [107].
Obesity makes surgical resection more difficult [63,108], and increases the risk of anal and perianal fistulas, as well as perioperative complications. Obesity and sarcopenia act synergistically in determining physical impairment and metabolic disorders, mobility, and mortality [61]. In addition, obesity seems to reduce the response to biologic therapy, especially the fixed-dosing drugs, by increasing their clearance, which makes weight loss an adjunctive therapy in obese IBD patients [109,110].
Since dietary and lifestyle modification often obtains only short-term results, in selected cases of severely obese patients, bariatric surgery has been proposed as safe and effective alternative [111].
3.5. Clinical Trials
While a large body of literature was published on new drugs for IBD in recent years, few data are available for nutritional clinical trials.
Low fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAP) diet is probably the most popular diet in functional gastrointestinal disorders, where it appears to be effective even in the long-term [112]. Some authors investigated its role even in the IBD setting. Bodini et al. [113] showed that six weeks of treatment with a low-FODMAP diet were able to reduce fecal calprotectin levels and to increase the quality of life of patients with IBD, probably related to the efficacy of low-FODMAP diet in reducing functional symptoms (like bloating, flatulence, or abdominal pain). In accordance, it is noteworthy that a randomized, double-blind, placebo-controlled, cross-over, and re-challenge trial conducted by Cox et al. [114] showed that fructans were able to exacerbate functional gastrointestinal symptoms in patients with IBD. These findings were confirmed in a subsequent, larger study aimed at evaluating the impact of low-FODMAP diet on symptoms, fecal microbiome and other markers of inflammation in IBD [115]: The low FODMAP diet reduced fecal abundance of microbes able to regulate the immune response, but had no significant effect on markers of inflammation; conversely, this diet induced significant improvements in functional symptom scores.
Another interesting clinical nutritional trial was conducted by Albenberg et al. [116], evaluating the impact of red meat consumption in patients with CD. Patients were divided in two groups according to the consumption of red meat (not more than 1 serving per month vs. a minimum of 2 servings per week) and followed for 49 weeks. Interestingly, the rates of CD relapses were similar in both groups.
4. Nutritional Therapy during Hospitalization
4.1. Hospitalization for Severe Disease
Nutritional interventions may improve outcomes for patients with IBD, especially in the most severe ones, and European Society for Clinical Nutrition and Metabolism (ESPEN) guidelines suggest screening for and manage undernutrition by using an appropriately trained multidisciplinary team [117]. Indeed, hospitalized patients with signs of malnutrition have higher risks of venous thromboembolism [118], non-elective surgery [119], longer hospital stay [119], and increased mortality [120]. Several indicators of malnutrition are currently accepted, but in clinical practice, the most used features are BMI < 18.5 kg/m2, unintentional weight loss exceeding 10% of total body weight and severe hypoalbuminaemia (<30 g/L) [121,122]. However, the American Society of Parenteral and Enteral Nutrition defines malnutrition as a status of any nutritional imbalance [123].
Initial findings of the importance of nutritional therapy were reported in the 1970s, when Voitk et al. showed an important clinical improvement of patients with severe CD treated with EEN [124,125]. EEN consists of either elemental, semi-elemental, or polymeric-based high-energy liquid formulas. An elemental diet is based on amino acids, sugars, fats, vitamins, and minerals, whereas non-elemental ones are composed of oligopeptide or whole-protein sources. Being amino acid-based, an elemental diet is completely absorbed in the duodenum and proximal jejunum, thus providing bowel rest for the distal small bowel and colon [126].
In children with active CD, EEN has been shown to be equally efficacious as steroids in order to induce clinical remission and mucosal healing, as well as to improve growth, correct micronutrient deficiencies, osteopenia, and anemia [127,128]. Therefore, it is suggested as a first-line therapy by ECCO/ESPGHAN guidelines [129]. The effect of EEN in inducing mucosal healing in IBD seems to be related primarily to modulation of gut microbiota [130,131], but some studies showed how it could be active in reducing pro-inflammatory cytokines [132,133].
On the other hand, in adult patients, enteral nutrition has lower effect and is less effective than corticosteroids in inducing remission [134,135]. However, the British Society of Gastroenterology suggests EEN for induction therapy in patients with mild-to-moderate CD who are interested in avoiding corticosteroids and are motivated to adhere strictly to EEN for up to 8 weeks [136]. Notably, a study by Heerasing et al. [137] in hospitalized patients with IBD showed that 25% of patients could avoid surgery due to EEN-induced remission. In Japan, EEN has been proposed as maintenance therapy in patients with CD, but its use in the USA and Europe is less widespread [138]. Interestingly, there is some evidence that the use of EEN in combination with infliximab could increase remission rates of response [139,140], and a meta-analysis showed that the combination of EEN with infliximab is more effective in maintaining clinical remission compared to infliximab alone (OR 2.7, CI 1.7–4.3) [141].
PN should be preferred in case of intestinal obstruction, intestinal ischemia, severe intestinal hemorrhage, high-output fistula, or ostomy, as well as in case of failure of enteral nutrition or inability to tolerate it [142,143]. Moreover, PN should be initiated even in case of severe malnutrition or short bowel syndrome due to previous bowel resections [144]. In the case of patients hospitalized for an acute inflammatory phase, particularly in the event of toxic megacolon and active fistulas, total parenteral nutrition (TPN) is the best option [145,146].
However, it is worth mentioning that PN, and especially long-term total parenteral nutrition (TPN), can be associated to infectious and thromboembolic complications (thrombophlebitis, deep venous thrombosis, and pulmonary embolism [147,148]). Patients with IBD are at risk for thromboembolism, which is related to multiple factors and includes an altered coagulation cascade, clinical factors, medications (steroids, tofacitinib), and surgery [149,150]. Notably, the risk of thromboembolism and infections related to PN in hospitalized patients is present even in pediatric ones [151]. Therefore, it is very important to start PN only in case of necessity.
Another important issue regarding hospitalized patients is fiber intake. We should report that a low-fiber diet is often ordered during hospitalization, but there are limited data in demonstrating its benefit. Nevertheless, the Academy of Nutrition and Dietetics and the American College of Gastroenterology recommends avoiding high-fiber foods in the presence of fistulas and strictures, as well as during CD flares [152,153]. The evidence about disease flares is more limited, although many patients note that high-fiber foods worsen IBD symptoms [154]. In the case of symptomatic strictures, it is logical to avoid high-fiber foods, which can induce a mechanical obstruction, though there are no data about the safe amount of fiber according to the extent of stricture [126].
4.2. Hospitalization for Surgery
Despite the use of biologic drugs, non-elective or elective IBD surgery rates have remained stable: Up to 70% of CD patients require abdominal surgery during their lifetime [155,156]. An optimal pre- and postoperative management can significantly improve the outcomes of patients who require surgery [157].
The most important problem related to nutrition in surgical patients is malnutrition. Indeed, the association between malnutrition and poor postoperative outcome was first reported in 1936, although not in IBD [158]. As expected, septic complications such as anastomotic leak, sepsis, and poor wound healing more frequently occur even in malnourished IBD patients [159]. A practical recommendation is to perform the Nutritional Risk Score (NRS) [160] and the Malnutrition Universal Screening Tool (MUST) [161] in all patients with IBD admitted to hospital for a surgical procedure. Patients with an NRS ≥ 3 have higher risks for complications after gastrointestinal surgery [162].
ECCO guidelines state that preoperative nutritional assessment should be performed for all patients with CD who need surgery [163]. Moreover, nutritional optimization prior to surgery, with enteral or parenteral nutrition, is recommended for those patients with nutritional deficiencies [163]. According to the ESPEN guidelines, the protocol for elective surgical patients includes avoidance of long-term fasting, integration of nutritional strategies into the overall management of the patient, metabolic homeostasis, and early mobilization [117]. Moreover, independently of the route of nutritional support, preoperative nutritional optimization in conjunction with stringent abdominal sepsis management and timely corticosteroid or immunosuppression withdrawal is able to avoid the need for a stoma in 92% of patients, with very few major complications and resulted in no postoperative mortality [164].
Although to correct nutritional imbalances is very important to increase postoperative outcome, no prospective randomized clinical trials have been conducted to assess the optimal route of nutritional supplementation before surgery, to the best of our knowledge.
It is worth noting that a depletion of arginine from surgical stress can impair wound healing, but it can be overcome by a preoperative supplementation [165]. Treatment with perioperative and postoperative immune-modulating nutrients, such as arginine or omega-3 fatty acids has been recommended either as supplements or included in foods, particularly for malnourished patients undergoing gastrointestinal surgery in order to reduce infective complications and to reduce the length of stay [166].
TPN is able to improve body weight, nutritional status, disease activity scores, and inflammatory markers in a study of Jacobson et al. [167], and clinical remission was achieved in all patients on TPN before surgery. More interestingly, no postoperative complications occurred within 30 days in the TPN group, compared with 27.6% in the matched controls [167]. Another study showed that preoperative TPN was associated with a longer hospital stay, but also with a reduced small-bowel resection length [168]. Some studies showed that TPN was able to improve BMI but not to reduce surgical complications [169,170].
A meta-analysis of Brennan et al. [171] showed preoperative EN and PN reduced postoperative complications, but EN reduced postoperative morbidity and should be preferred as nutritional support, while PN should be reserved for those patients who are unable to tolerate EN. Indeed, the above-mentioned interesting study by Zerbib et al. [164] displayed that EEN was more effective in ameliorating nutritional parameters, such as serum albumin levels or, more importantly, in reducing the rate of intra-abdominal septic complications up to 3 months postoperatively. Accordingly, these results were confirmed in three Asian studies [172,173,174], which showed an effect of EEN in improving BMI, hemoglobin, albumin, and CRP levels, as well as in reducing the rates of infections and anastomotic leak. The lower rate of complications in patients treated preoperatively with EEN was previously demonstrated even by Smedh et al. [175], which compared a cohort treated with EEN with a retrospective cohort without this management, highlighting how the EEN-treated patients displayed lower surgical complications in the first 30 days. Furthermore, the study by Heerasing et al. [137] showed that activation of an EEN protocol avoided surgery in 25% of complicated CD patients.
For these reasons, ECCO guidelines suggest that a goal-driven PN should be considered only whenever EEN is difficult [163]. A summary of the study evaluating EEN and PN in surgical patients with IBD is displayed in Table 2.
It is worth mentioning the role of diet following surgery in IBD. Current guidelines suggest that patients should avoid fibers in the perioperative period [159]. However, a large survey conducted in North America showed that the avoidance of fiber is associated with a greater risk of CD flare in a 6-month period [43]. Therefore, we suggest the reduction of fiber intake only in the initial weeks following surgery, with a gradual reintroduction.
In patients with ileal pouch after colectomy, the intake of antioxidants (such as cryptoxanthin, lycopene, vitamin A and vitamin C), mainly found in fruits, seem to reduce the rates of pouchitis [176,177]. This correlation could be explained by the modification in gut microbiota induced by the assumption of a higher quantity of antioxidants [177]. Accordingly, an adherence to the Mediterranean diet is associated with decreased fecal calprotectin levels after pouch surgery [178].
5. Conclusions
In the last decades, nutritional issues in IBD have received growing attention, focused on the pathogenic role of the western diet, as well as on the therapeutic impact of nutrition. Westernized dietary patterns have been recognized as risk factors for IBD because they promote a dysbiotic profile and intestinal inflammation [15].
In active disease, the accurate assessment of nutritional status, including sarcopenia and obesity, as well as the early treatment of protein/energy malnutrition and micronutrient/vitamin deficiencies, are strongly recommended [61]. Several nutritional strategies have been explored as exclusive (EEN for pediatric CD) or add-on therapy. PN is indicated only when enteral nutrition has failed or is contraindicated, particularly in hospitalized patients and in pre- and postoperative settings. The assessment and the treatment of malnutrition are crucial to improve the therapeutic outcome and prevent complications [117]. Therefore, the best therapy of IBD cannot be separated from an adequate nutritional management.
Figure 2 displays our conclusions.
Author Contributions
Conceptualization, F.C. and M.G.M.; writing—original draft preparation, L.B. and D.G.R.; writing—review and editing, M.G.M., M.B. and F.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This paper is a review, and therefore do not have data to show.
Conflicts of Interest
Francesco Costa received Board Membership honoraria from Takeda, Janssen, Amgen, and lecture fees from Abbvie, Takeda, Zambon, Ferring, Diasorin, Otsuka, and MSD; none of these honoraria had an influence on this paper. Davide Giuseppe Ribaldone received Board Membership honoraria from Abbvie and Janssen, and lecture fees from Janssen; none of these honoraria had an influence on this paper. Other authors have no conflict of interest to declare.
References
- Fiocchi, C. Inflammatory Bowel Disease: Complexity and Variability Need Integration. Front. Med. 2018, 5, 75. [Google Scholar] [CrossRef] [PubMed]
- Chapman-Kiddell, C.A.; Davies, P.S.; Gillen, L.; Radford-Smith, G.L. Role of diet in the development of inflammatory bowel disease. Inflamm. Bowel Dis. 2010, 16, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Lopez, S. Epidemiology, follow-up, monitoring and other aspects of inflammatory bowel disease. Gastroenterol. Hepatol. 2015, 38 (Suppl. 1), 32–38. [Google Scholar] [CrossRef]
- Park, J.; Cheon, J.H. Incidence and Prevalence of Inflammatory Bowel Disease across Asia. Yonsei Med. J. 2021, 62, 99–108. [Google Scholar] [CrossRef]
- Schreiner, P.; Martinho-Grueber, M.; Studerus, D.; Vavricka, S.R.; Tilg, H.; Biedermann, L.; on behalf of Swiss Ibdnet, an Official Working Group of the Swiss Society of Gastroenterology. Nutrition in Inflammatory Bowel Disease. Digestion 2020, 101 (Suppl. 1), 120–135. [Google Scholar] [CrossRef]
- Holt, D.Q.; Strauss, B.J.; Moore, G.T. Patients with inflammatory bowel disease and their treating clinicians have different views regarding diet. J. Hum. Nutr. Diet. Off. J. Br. Diet. Assoc. 2017, 30, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Casanova, M.J.; Chaparro, M.; Molina, B.; Merino, O.; Batanero, R.; Duenas-Sadornil, C.; Robledo, P.; Garcia-Albert, A.M.; Gomez-Sanchez, M.B.; Calvet, X.; et al. Prevalence of Malnutrition and Nutritional Characteristics of Patients with Inflammatory Bowel Disease. J. Crohn's Colitis 2017, 11, 1430–1439. [Google Scholar] [CrossRef]
- Zallot, C.; Quilliot, D.; Chevaux, J.B.; Peyrin-Biroulet, C.; Gueant-Rodriguez, R.M.; Freling, E.; Collet-Fenetrier, B.; Williet, N.; Ziegler, O.; Bigard, M.A.; et al. Dietary beliefs and behavior among inflammatory bowel disease patients. Inflamm. Bowel Dis. 2013, 19, 66–72. [Google Scholar] [CrossRef]
- De Vries, J.H.M.; Dijkhuizen, M.; Tap, P.; Witteman, B.J.M. Patient's Dietary Beliefs and Behaviours in Inflammatory Bowel Disease. Dig. Dis. 2019, 37, 131–139. [Google Scholar] [CrossRef]
- Cashman, K.D.; Shanahan, F. Is nutrition an aetiological factor for inflammatory bowel disease? Eur. J. Gastroenterol. Hepatol. 2003, 15, 607–613. [Google Scholar] [CrossRef]
- Campos, F.G.; Waitzberg, D.L.; Teixeira, M.G.; Mucerino, D.R.; Kiss, D.R.; Habr-Gama, A. Pharmacological nutrition in inflammatory bowel diseases. Nutr. Hosp. 2003, 18, 57–64. [Google Scholar]
- Bertani, L.; Mumolo, M.G.; Tapete, G.; Albano, E.; Baiano Svizzero, G.; Zanzi, F.; Ceccarelli, L.; Bellini, M.; Marchi, S.; Costa, F. Fecal calprotectin: Current and future perspectives for inflammatory bowel disease treatment. Eur. J. Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef]
- Carter, M.J.; Lobo, A.J.; Travis, S.P.; Ibd Section, B.S. Guidelines for the management of inflammatory bowel disease in adults. Gut 2004, 53 (Suppl. 5), V1–V16. [Google Scholar] [CrossRef]
- Ko, Y. Inflammatory bowel disease environmental risk factors versus genetics based on migration epidemiological studies. J. Gastroenterol. Hepatol. 2018, 33 (Suppl. 3), 22. [Google Scholar] [CrossRef]
- Mentella, M.C.; Scaldaferri, F.; Pizzoferrato, M.; Gasbarrini, A.; Miggiano, G.A.D. Nutrition, IBD and Gut Microbiota: A Review. Nutrients 2020, 12, 944. [Google Scholar] [CrossRef] [PubMed]
- Devkota, S.; Wang, Y.; Musch, M.W.; Leone, V.; Fehlner-Peach, H.; Nadimpalli, A.; Antonopoulos, D.A.; Jabri, B.; Chang, E.B. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature 2012, 487, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Muhomah, T.A.; Nishino, N.; Katsumata, E.; Haoming, W.; Tsuruta, T. High-fat diet reduces the level of secretory immunoglobulin A coating of commensal gut microbiota. Biosci. Microbiotafood Health 2019, 38, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Barnes, E.L.; Nestor, M.; Onyewadume, L.; de Silva, P.S.; Korzenik, J.R.; Investigators, D. High Dietary Intake of Specific Fatty Acids Increases Risk of Flares in Patients with Ulcerative Colitis in Remission during Treatment with Aminosalicylates. Clin. Gastroenterol. Hepatol. 2017, 15, 1390–1396. [Google Scholar] [CrossRef] [PubMed]
- John, S.; Luben, R.; Shrestha, S.S.; Welch, A.; Khaw, K.T.; Hart, A.R. Dietary n-3 polyunsaturated fatty acids and the aetiology of ulcerative colitis: A UK prospective cohort study. Eur. J. Gastroenterol. Hepatol. 2010, 22, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; Khalili, H.; Konijeti, G.G.; Higuchi, L.M.; de Silva, P.; Fuchs, C.S.; Willett, W.C.; Richter, J.M.; Chan, A.T. Long-term intake of dietary fat and risk of ulcerative colitis and Crohn's disease. Gut 2014, 63, 776–784. [Google Scholar] [CrossRef]
- Amre, D.K.; D'Souza, S.; Morgan, K.; Seidman, G.; Lambrette, P.; Grimard, G.; Israel, D.; Mack, D.; Ghadirian, P.; Deslandres, C.; et al. Imbalances in dietary consumption of fatty acids, vegetables, and fruits are associated with risk for Crohn's disease in children. Am. J. Gastroenterol. 2007, 102, 2016–2025. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Han, T.J.; Liu, J.; Li, J.S.; Zhang, X.H.; Wang, Y.; Li, Q.Y.; Zhu, Q.; Yang, C.M. Meat intake and risk of inflammatory bowel disease: A meta-analysis. Turk. J. Gastroenterol. 2015, 26, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Lewin, M.H.; Bailey, N.; Bandaletova, T.; Bowman, R.; Cross, A.J.; Pollock, J.; Shuker, D.E.; Bingham, S.A. Red meat enhances the colonic formation of the DNA adduct O6-carboxymethyl guanine: Implications for colorectal cancer risk. Cancer Res. 2006, 66, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Le Leu, R.K.; Young, G.P.; Hu, Y.; Winter, J.; Conlon, M.A. Dietary red meat aggravates dextran sulfate sodium-induced colitis in mice whereas resistant starch attenuates inflammation. Dig. Dis. Sci. 2013, 58, 3475–3482. [Google Scholar] [CrossRef]
- Jantchou, P.; Morois, S.; Clavel-Chapelon, F.; Boutron-Ruault, M.C.; Carbonnel, F. Animal protein intake and risk of inflammatory bowel disease: The E3N prospective study. Am. J. Gastroenterol. 2010, 105, 2195–2201. [Google Scholar] [CrossRef]
- Jowett, S.L.; Seal, C.J.; Pearce, M.S.; Phillips, E.; Gregory, W.; Barton, J.R.; Welfare, M.R. Influence of dietary factors on the clinical course of ulcerative colitis: A prospective cohort study. Gut 2004, 53, 1479–1484. [Google Scholar] [CrossRef]
- Lee, G.; Han, J.H.; Maeng, H.J.; Lim, S. Three-Month Daily Consumption of Sugar-Sweetened Beverages Affects the Liver, Adipose Tissue, and Glucose Metabolism. J. Obes. Metab. Syndr. 2020, 29, 26–38. [Google Scholar] [CrossRef]
- Martini, G.A.; Brandes, J.W. Increased consumption of refined carbohydrates in patients with Crohn's disease. Klin. Wochenschr. 1976, 54, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Jarnerot, G.; Jarnmark, I.; Nilsson, K. Consumption of refined sugar by patients with Crohn's disease, ulcerative colitis, or irritable bowel syndrome. Scand. J. Gastroenterol. 1983, 18, 999–1002. [Google Scholar] [CrossRef]
- Mayberry, J.F.; Rhodes, J.; Newcombe, R.G. Increased sugar consumption in Crohn's disease. Digestion 1980, 20, 323–326. [Google Scholar] [CrossRef]
- Matsui, T.; Iida, M.; Fujishima, M.; Imai, K.; Yao, T. Increased sugar consumption in Japanese patients with Crohn's disease. Gastroenterol. Jpn. 1990, 25, 271. [Google Scholar] [CrossRef]
- Khan, S.; Waliullah, S.; Godfrey, V.; Khan, M.A.W.; Ramachandran, R.A.; Cantarel, B.L.; Behrendt, C.; Peng, L.; Hooper, L.V.; Zaki, H. Dietary simple sugars alter microbial ecology in the gut and promote colitis in mice. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef]
- Hiippala, K.; Kainulainen, V.; Kalliomaki, M.; Arkkila, P.; Satokari, R. Mucosal Prevalence and Interactions with the Epithelium Indicate Commensalism of Sutterella spp. Front. Microbiol. 2016, 7, 1706. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.N.; St Amand, A.L.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef] [PubMed]
- Vernero, M.; De Blasio, F.; Ribaldone, D.G.; Bugianesi, E.; Pellicano, R.; Saracco, G.M.; Astegiano, M.; Caviglia, G.P. The Usefulness of Microencapsulated Sodium Butyrate Add-On Therapy in Maintaining Remission in Patients with Ulcerative Colitis: A Prospective Observational Study. J. Clin. Med. 2020, 9, 3941. [Google Scholar] [CrossRef]
- Goncalves, P.; Araujo, J.R.; Di Santo, J.P. A Cross-Talk between Microbiota-Derived Short-Chain Fatty Acids and the Host Mucosal Immune System Regulates Intestinal Homeostasis and Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2018, 24, 558–572. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; Khalili, H.; Konijeti, G.G.; Higuchi, L.M.; de Silva, P.; Korzenik, J.R.; Fuchs, C.S.; Willett, W.C.; Richter, J.M.; Chan, A.T. A prospective study of long-term intake of dietary fiber and risk of Crohn's disease and ulcerative colitis. Gastroenterology 2013, 145, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; Khalili, H.; Song, M.; Higuchi, L.M.; Richter, J.M.; Nimptsch, K.; Wu, K.; Chan, A.T. High School Diet and Risk of Crohn's Disease and Ulcerative Colitis. Inflamm. Bowel Dis. 2015, 21, 2311–2319. [Google Scholar] [CrossRef]
- Andersen, V.; Olsen, A.; Carbonnel, F.; Tjonneland, A.; Vogel, U. Diet and risk of inflammatory bowel disease. Dig. Liver Dis. 2012, 44, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Andersen, V.; Chan, S.; Luben, R.; Khaw, K.T.; Olsen, A.; Tjonneland, A.; Kaaks, R.; Grip, O.; Bergmann, M.M.; Boeing, H.; et al. Fibre intake and the development of inflammatory bowel disease: A European prospective multi-centre cohort study (EPIC-IBD). J. Crohn's Colitis 2018, 12, 129–136. [Google Scholar] [CrossRef]
- Milajerdi, A.; Ebrahimi-Daryani, N.; Dieleman, L.A.; Larijani, B.; Esmaillzadeh, A. Association of Dietary Fiber, Fruit, and Vegetable Consumption with Risk of Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Adv. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Brotherton, C.S.; Martin, C.A.; Long, M.D.; Kappelman, M.D.; Sandler, R.S. Avoidance of Fiber Is Associated with Greater Risk of Crohn's Disease Flare in a 6-Month Period. Clin. Gastroenterol. Hepatol. 2016, 14, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Hayes, D.; Angove, M.J.; Tucci, J.; Dennis, C. Walnuts (Juglans regia) Chemical Composition and Research in Human Health. Crit. Rev. Food Sci. Nutr. 2016, 56, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, M.; Matz, A.; Klemashevich, C.; Rosenberg, D.W. Dietary Walnut Supplementation Alters Mucosal Metabolite Profiles during DSS-Induced Colonic Ulceration. Nutrients 2019, 11, 1118. [Google Scholar] [CrossRef]
- Meza-Meza, M.R.; Ruiz-Ballesteros, A.I.; de la Cruz-Mosso, U. Functional effects of vitamin D: From nutrient to immunomodulator. Crit. Rev. Food Sci. Nutr. 2020, 1–21. [Google Scholar] [CrossRef]
- Domislovic, V.; Vranesic Bender, D.; Barisic, A.; Brinar, M.; Ljubas Kelecic, D.; Rotim, C.; Novosel, M.; Matasin, M.; Krznaric, Z. High Prevalence of Untreated and Undertreated Vitamin D Deficiency and Insufficiency in Patients with Inflammatory Bowel Disease. Acta Clin. Croat. 2020, 59, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.G.; Wu, S.; Sun, J. Vitamin D, Vitamin D Receptor, and Tissue Barriers. Tissue Barriers 2013, 1. [Google Scholar] [CrossRef]
- Trochoutsou, A.I.; Kloukina, V.; Samitas, K.; Xanthou, G. Vitamin-D in the Immune System: Genomic and Non-Genomic Actions. Mini Rev. Med. Chem. 2015, 15, 953–963. [Google Scholar] [CrossRef]
- Penna, G.; Adorini, L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J. Immunol. 2000, 164, 2405–2411. [Google Scholar] [CrossRef]
- Ooi, J.H.; Li, Y.; Rogers, C.J.; Cantorna, M.T. Vitamin D regulates the gut microbiome and protects mice from dextran sodium sulfate-induced colitis. J. Nutr. 2013, 143, 1679–1686. [Google Scholar] [CrossRef]
- Lagishetty, V.; Misharin, A.V.; Liu, N.Q.; Lisse, T.S.; Chun, R.F.; Ouyang, Y.; McLachlan, S.M.; Adams, J.S.; Hewison, M. Vitamin D deficiency in mice impairs colonic antibacterial activity and predisposes to colitis. Endocrinology 2010, 151, 2423–2432. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Cagan, A.; Gainer, V.S.; Cai, T.; Cheng, S.C.; Savova, G.; Chen, P.; Szolovits, P.; Xia, Z.; De Jager, P.L.; et al. Normalization of plasma 25-hydroxy vitamin D is associated with reduced risk of surgery in Crohn's disease. Inflamm. Bowel Dis. 2013, 19, 1921–1927. [Google Scholar] [CrossRef] [PubMed]
- Ulitsky, A.; Ananthakrishnan, A.N.; Naik, A.; Skaros, S.; Zadvornova, Y.; Binion, D.G.; Issa, M. Vitamin D deficiency in patients with inflammatory bowel disease: Association with disease activity and quality of life. J. Parenter. Enter. Nutr. 2011, 35, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Ham, N.S.; Hwang, S.W.; Oh, E.H.; Kim, J.; Lee, H.S.; Park, S.H.; Yang, D.H.; Ye, B.D.; Byeon, J.S.; Myung, S.J.; et al. Influence of Severe Vitamin D Deficiency on the Clinical Course of Inflammatory Bowel Disease. Dig. Dis. Sci. 2021, 66, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.L.; Keita, A.V.; Duncan, S.H.; O'Kennedy, N.; Soderholm, J.D.; Rhodes, J.M.; Campbell, B.J. Translocation of Crohn's disease Escherichia coli across M-cells: Contrasting effects of soluble plant fibres and emulsifiers. Gut 2010, 59, 1331–1339. [Google Scholar] [CrossRef]
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015, 519, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Hebuterne, X.; Filippi, J.; Schneider, S.M. Nutrition in adult patients with inflammatory bowel disease. Curr. Drug Targets 2014, 15, 1030–1038. [Google Scholar] [CrossRef]
- Balestrieri, P.; Ribolsi, M.; Guarino, M.P.L.; Emerenziani, S.; Altomare, A.; Cicala, M. Nutritional Aspects in Inflammatory Bowel Diseases. Nutrients 2020, 12, 372. [Google Scholar] [CrossRef]
- Hwang, C.; Ross, V.; Mahadevan, U. Micronutrient deficiencies in inflammatory bowel disease: From A to zinc. Inflamm. Bowel Dis. 2012, 18, 1961–1981. [Google Scholar] [CrossRef]
- Scaldaferri, F.; Pizzoferrato, M.; Lopetuso, L.R.; Musca, T.; Ingravalle, F.; Sicignano, L.L.; Mentella, M.; Miggiano, G.; Mele, M.C.; Gaetani, E.; et al. Nutrition and IBD: Malnutrition and/or Sarcopenia? A Practical Guide. Gastroenterol. Res. Pract. 2017, 2017, 8646495. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ney, M.; Eslamparast, T.; Vandermeer, B.; Ismond, K.P.; Kroeker, K.; Halloran, B.; Raman, M.; Tandon, P. Systematic review of nutrition screening and assessment in inflammatory bowel disease. World J. Gastroenterol. 2019, 25, 3823–3837. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Dulai, P.S.; Zarrinpar, A.; Ramamoorthy, S.; Sandborn, W.J. Obesity in IBD: Epidemiology, pathogenesis, disease course and treatment outcomes. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Yueying, C.; Yu Fan, W.; Jun, S. Anemia and iron deficiency in Crohn's disease. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 155–162. [Google Scholar] [CrossRef]
- Yakut, M.; Ustun, Y.; Kabacam, G.; Soykan, I. Serum vitamin B12 and folate status in patients with inflammatory bowel diseases. Eur. J. Intern. Med. 2010, 21, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Duerksen, D.R.; Fallows, G.; Bernstein, C.N. Vitamin B12 malabsorption in patients with limited ileal resection. Nutrition 2006, 22, 1210–1213. [Google Scholar] [CrossRef]
- Hlavaty, T.; Krajcovicova, A.; Payer, J. Vitamin D therapy in inflammatory bowel diseases: Who, in what form, and how much? J. Crohn's Colitis 2015, 9, 198–209. [Google Scholar] [CrossRef]
- Ghishan, F.K.; Kiela, P.R. Vitamins and Minerals in Inflammatory Bowel Disease. Gastroenterol. Clin. N. Am. 2017, 46, 797–808. [Google Scholar] [CrossRef]
- Vagianos, K.; Bector, S.; McConnell, J.; Bernstein, C.N. Nutrition assessment of patients with inflammatory bowel disease. J. Parenter. Enter. Nutr. 2007, 31, 311–319. [Google Scholar] [CrossRef]
- Rogler, G.; Vavricka, S. Anemia in inflammatory bowel disease: An under-estimated problem? Front. Med. 2014, 1, 58. [Google Scholar] [CrossRef]
- Thomas, D.W.; Hinchliffe, R.F.; Briggs, C.; Macdougall, I.C.; Littlewood, T.; Cavill, I.; British Committee for Standards in Haematology. Guideline for the laboratory diagnosis of functional iron deficiency. Br. J. Haematol. 2013, 161, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, K.M.; Gasche, C. Management of Iron Deficiency Anaemia in Inflammatory Bowel Disease. Acta Haematol. 2019, 142, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Bonovas, S.; Fiorino, G.; Allocca, M.; Lytras, T.; Tsantes, A.; Peyrin-Biroulet, L.; Danese, S. Intravenous Versus Oral Iron for the Treatment of Anemia in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicine 2016, 95, e2308. [Google Scholar] [CrossRef] [PubMed]
- Vernero, M.; Boano, V.; Ribaldone, D.G.; Pellicano, R.; Astegiano, M. Oral iron supplementation with Feralgine(R) in inflammatory bowel disease: A retrospective observational study. Minerva Gastroenterol. E Dietol. 2019, 65, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Bertani, L.; Tricò, D.; Zanzi, F.; Baiano Svizzero, G.; Coppini, F.; de Bortoli, N.; Bellini, M.; Antonioli, L.; Blandizzi, C.; Marchi, S. Oral sucrosomial iron is as effective as intravenous ferric carboxy-maltose in treating anemia in patients with ulcerative colitis. Nutrients 2021, 13, 608. [Google Scholar] [CrossRef]
- MacMaster, M.J.; Damianopoulou, S.; Thomson, C.; Talwar, D.; Stefanowicz, F.; Catchpole, A.; Gerasimidis, K.; Gaya, D.R. A prospective analysis of micronutrient status in quiescent inflammatory bowel disease. Clin. Nutr. 2021, 40, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Liu, Y.; Guo, H.; Jabir, M.S.; Liu, X.; Cui, W.; Li, D. Associations between Folate and Vitamin B12 Levels and Inflammatory Bowel Disease: A Meta-Analysis. Nutrients 2017, 9, 382. [Google Scholar] [CrossRef]
- Damoiseaux, M.; van Doorn, W.; van Lochem, E.; Damoiseaux, J. Testing for IgA anti-tissue transglutaminase in routine clinical practice: Requesting behaviour in relation to prevalence of positive results. J. Transl. Autoimmun. 2020, 3, 100045. [Google Scholar] [CrossRef]
- Al Mutairi, F. Hyperhomocysteinemia: Clinical Insights. J. Cent. Nerv. Syst. Dis. 2020, 12, 1179573520962230. [Google Scholar] [CrossRef]
- Cammarota, T.; Ribaldone, D.G.; Resegotti, A.; Repici, A.; Danese, S.; Fiorino, G.; Sarno, A.; Robotti, D.; Debani, P.; Bonenti, G.; et al. Role of bowel ultrasound as a predictor of surgical recurrence of Crohn's disease. Scand. J. Gastroenterol. 2013, 48, 552–555. [Google Scholar] [CrossRef]
- Cordaro, M.; Siracusa, R.; Fusco, R.; Cuzzocrea, S.; Di Paola, R.; Impellizzeri, D. Involvements of Hyperhomocysteinemia in Neurological Disorders. Metabolites 2021, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Tugba-Kartal, A.; Cagla-Mutlu, Z. Comparison of Sublingual and Intramuscular Administration of Vitamin B12 for the Treatment of Vitamin B12 Deficiency in Children. Rev. Investig. Clin. Organo Hosp. Enferm. Nutr. 2020, 72, 380–385. [Google Scholar] [CrossRef]
- Mentella, M.C.; Scaldaferri, F.; Pizzoferrato, M.; Gasbarrini, A.; Miggiano, G.A.D. The Association of Disease Activity, BMI and Phase Angle with Vitamin D Deficiency in Patients with IBD. Nutrients 2019, 11, 2583. [Google Scholar] [CrossRef] [PubMed]
- Saraff, V.; Shaw, N. Sunshine and vitamin D. Arch. Dis. Child. 2016, 101, 190–192. [Google Scholar] [CrossRef]
- Chang, S.W.; Lee, H.C. Vitamin D and health—The missing vitamin in humans. Pediatr. Neonatol. 2019, 60, 237–244. [Google Scholar] [CrossRef]
- Cesareo, R.; Attanasio, R.; Caputo, M.; Castello, R.; Chiodini, I.; Falchetti, A.; Guglielmi, R.; Papini, E.; Santonati, A.; Scillitani, A.; et al. Italian Association of Clinical Endocrinologists (AME) and Italian Chapter of the American Association of Clinical Endocrinologists (AACE) Position Statement: Clinical Management of Vitamin D Deficiency in Adults. Nutrients 2018, 10, 546. [Google Scholar] [CrossRef]
- Pearce, S.H.; Cheetham, T.D. Diagnosis and management of vitamin D deficiency. BMJ 2010, 340, b5664. [Google Scholar] [CrossRef]
- Gilca-Blanariu, G.E.; Diaconescu, S.; Ciocoiu, M.; Stefanescu, G. New Insights into the Role of Trace Elements in IBD. BioMed Res. Int. 2018, 2018, 1813047. [Google Scholar] [CrossRef] [PubMed]
- Wieringa, F.T.; Dijkhuizen, M.A.; Fiorentino, M.; Laillou, A.; Berger, J. Determination of zinc status in humans: Which indicator should we use? Nutrients 2015, 7, 3252–3263. [Google Scholar] [CrossRef]
- Siva, S.; Rubin, D.T.; Gulotta, G.; Wroblewski, K.; Pekow, J. Zinc Deficiency is Associated with Poor Clinical Outcomes in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 152–157. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Khalili, H.; Song, M.; Higuchi, L.M.; Richter, J.M.; Chan, A.T. Zinc intake and risk of Crohn's disease and ulcerative colitis: A prospective cohort study. Int. J. Epidemiol. 2015, 44, 1995–2005. [Google Scholar] [CrossRef] [PubMed]
- Ribaldone, D.G.; Pellicano, R.; Actis, G.C. Inflammation in gastrointestinal disorders: Prevalent socioeconomic factors. Clin. Exp. Gastroenterol. 2019, 12, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Massironi, S.; Cavalcoli, F.; Rausa, E.; Invernizzi, P.; Braga, M.; Vecchi, M. Understanding short bowel syndrome: Current status and future perspectives. Dig. Liver Dis. 2020, 52, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Limketkai, B.N.; Iheozor-Ejiofor, Z.; Gjuladin-Hellon, T.; Parian, A.; Matarese, L.E.; Bracewell, K.; MacDonald, J.K.; Gordon, M.; Mullin, G.E. Dietary interventions for induction and maintenance of remission in inflammatory bowel disease. Cochrane Database Syst. Rev. 2019, 2, CD012839. [Google Scholar] [CrossRef] [PubMed]
- Kakodkar, S.; Mutlu, E.A. Diet as a Therapeutic Option for Adult Inflammatory Bowel Disease. Gastroenterol. Clin. N. Am. 2017, 46, 745–767. [Google Scholar] [CrossRef]
- Agin, M.; Yucel, A.; Gumus, M.; Yuksekkaya, H.A.; Tumgor, G. The Effect of Enteral Nutrition Support Rich in TGF-Beta in the Treatment of Inflammatory Bowel Disease in Childhood. Medicina 2019, 55, 620. [Google Scholar] [CrossRef]
- Shafiee, N.H.; Manaf, Z.A.; Mokhtar, N.M.; Raja Ali, R.A. Anti-inflammatory diet and inflammatory bowel disease: What clinicians and patients should know? Intest. Res. 2021. [Google Scholar] [CrossRef]
- Jo, E.; Lee, S.R.; Park, B.S.; Kim, J.S. Potential mechanisms underlying the role of chronic inflammation in age-related muscle wasting. Aging Clin. Exp. Res. 2012, 24, 412–422. [Google Scholar] [CrossRef]
- Ryan, E.; McNicholas, D.; Creavin, B.; Kelly, M.E.; Walsh, T.; Beddy, D. Sarcopenia and Inflammatory Bowel Disease: A Systematic Review. Inflamm. Bowel Dis. 2019, 25, 67–73. [Google Scholar] [CrossRef]
- Watanabe, H.; Enoki, Y.; Maruyama, T. Sarcopenia in Chronic Kidney Disease: Factors, Mechanisms, and Therapeutic Interventions. Biol. Pharm. Bull. 2019, 42, 1437–1445. [Google Scholar] [CrossRef]
- Zhang, T.; Cao, L.; Cao, T.; Yang, J.; Gong, J.; Zhu, W.; Li, N.; Li, J. Prevalence of Sarcopenia and Its Impact on Postoperative Outcome in Patients with Crohn's Disease Undergoing Bowel Resection. J. Parenter. Enter. Nutr. 2017, 41, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Skrzypczak, D.; Ratajczak, A.E.; Szymczak-Tomczak, A.; Dobrowolska, A.; Eder, P.; Krela-Kazmierczak, I. A Vicious Cycle of Osteosarcopeniain Inflammatory Bowel Diseases-Aetiology, Clinical Implications and Therapeutic Perspectives. Nutrients 2021, 13, 293. [Google Scholar] [CrossRef] [PubMed]
- Bryant, R.V.; Trott, M.J.; Bartholomeusz, F.D.; Andrews, J.M. Systematic review: Body composition in adults with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2013, 38, 213–225. [Google Scholar] [CrossRef]
- Bryant, R.V.; Ooi, S.; Schultz, C.G.; Goess, C.; Grafton, R.; Hughes, J.; Lim, A.; Bartholomeusz, F.D.; Andrews, J.M. Low muscle mass and sarcopenia: Common and predictive of osteopenia in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2015, 41, 895–906. [Google Scholar] [CrossRef]
- Bertani, L.; Trico, D.; Pugliese, D.; Privitera, G.; Linsalata, G.; Zanzi, F.; Gloria Mumolo, M.; Barberio, B.; Monzani, F.; Marchi, S.; et al. Serum triiodothyronine-to-thyroxine (T3/T4) ratio predicts therapeutic outcome to biological therapies in elderly IBD patients. Aliment. Pharmacol. Ther. 2021, 53, 273–280. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef]
- Steed, H.; Walsh, S.; Reynolds, N. A brief report of the epidemiology of obesity in the inflammatory bowel disease population of Tayside, Scotland. Obes. Facts 2009, 2, 370–372. [Google Scholar] [CrossRef]
- Hicks, G.; Abdulaal, A.; Slesser, A.A.P.; Mohsen, Y. Outcomes of inflammatory bowel disease surgery in obese versus non-obese patients: A meta-analysis. Tech. Coloproctol. 2019, 23, 947–955. [Google Scholar] [CrossRef]
- Singh, S.; Picardo, S.; Seow, C.H. Management of Inflammatory Bowel Diseases in Special Populations: Obese, Old, or Obstetric. Clin. Gastroenterol. Hepatol. 2020, 18, 1367–1380. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.W.; Sinanan, M.N.; Zisman, T.L. Increased body mass index is associated with earlier time to loss of response to infliximab in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2013, 19, 2118–2124. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.L.; Barnes, E.L.; Herfarth, H.H.; Isaacs, K.L.; Jain, A. Bariatric Surgery Is a Safe and Effective Option for Patients with Inflammatory Bowel Diseases: A Case Series and Systematic Review of the Literature. Inflamm. Intest. Dis. 2019, 3, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.; Tonarelli, S.; Barracca, F.; Morganti, R.; Pancetti, A.; Bertani, L.; de Bortoli, N.; Costa, F.; Mosca, M.; Marchi, S.; et al. A Low-FODMAP Diet for Irritable Bowel Syndrome: Some Answers to the Doubts from a Long-Term Follow-Up. Nutrients 2020, 12, 2360. [Google Scholar] [CrossRef] [PubMed]
- Bodini, G.; Zanella, C.; Crespi, M.; Lo Pumo, S.; Demarzo, M.G.; Savarino, E.; Savarino, V.; Giannini, E.G. A randomized, 6-wk trial of a low FODMAP diet in patients with inflammatory bowel disease. Nutrition 2019, 67–68, 110542. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.R.; Prince, A.C.; Myers, C.E.; Irving, P.M.; Lindsay, J.O.; Lomer, M.C.; Whelan, K. Fermentable Carbohydrates [FODMAPs] Exacerbate Functional Gastrointestinal Symptoms in Patients with Inflammatory Bowel Disease: A Randomised, Double-blind, Placebo-controlled, Cross-over, Re-challenge Trial. J. Crohn's Colitis 2017, 11, 1420–1429. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.R.; Lindsay, J.O.; Fromentin, S.; Stagg, A.J.; McCarthy, N.E.; Galleron, N.; Ibraim, S.B.; Roume, H.; Levenez, F.; Pons, N.; et al. Effects of Low FODMAP Diet on Symptoms, Fecal Microbiome, and Markers of Inflammation in Patients with Quiescent Inflammatory Bowel Disease in a Randomized Trial. Gastroenterology 2020, 158, 176–188. [Google Scholar] [CrossRef]
- Albenberg, L.; Brensinger, C.M.; Wu, Q.; Gilroy, E.; Kappelman, M.D.; Sandler, R.S.; Lewis, J.D. A Diet Low in Red and Processed Meat Does Not Reduce Rate of Crohn's Disease Flares. Gastroenterology 2019, 157, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Escher, J.; Hebuterne, X.; Klek, S.; Krznaric, Z.; Schneider, S.; Shamir, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN practical guideline: Clinical Nutrition in inflammatory bowel disease. Clin. Nutr. 2020, 39, 632–653. [Google Scholar] [CrossRef]
- Wallaert, J.B.; De Martino, R.R.; Marsicovetere, P.S.; Goodney, P.P.; Finlayson, S.R.; Murray, J.J.; Holubar, S.D. Venous thromboembolism after surgery for inflammatory bowel disease: Are there modifiable risk factors? Data from ACS NSQIP. Dis. Colon Rectum 2012, 55, 1138–1144. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; McGinley, E.L.; Binion, D.G.; Saeian, K. A novel risk score to stratify severity of Crohn's disease hospitalizations. Am. J. Gastroenterol. 2010, 105, 1799–1807. [Google Scholar] [CrossRef]
- Nguyen, G.C.; Munsell, M.; Harris, M.L. Nationwide prevalence and prognostic significance of clinically diagnosable protein-calorie malnutrition in hospitalized inflammatory bowel disease patients. Inflamm. Bowel Dis. 2008, 14, 1105–1111. [Google Scholar] [CrossRef]
- Lindor, K.D.; Fleming, C.R.; Ilstrup, D.M. Preoperative nutritional status and other factors that influence surgical outcome in patients with Crohn's disease. Mayo Clin. Proc. 1985, 60, 393–396. [Google Scholar] [CrossRef]
- Donnellan, C.F.; Yann, L.H.; Lal, S. Nutritional management of Crohn's disease. Ther. Adv. Gastroenterol. 2013, 6, 231–242. [Google Scholar] [CrossRef]
- White, J.V.; Guenter, P.; Jensen, G.; Malone, A.; Schofield, M.; Academy Malnutrition Work Group; Malnutrition Task Force; Board of Directors; Dietetics Malnutrition Work. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: Characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J. Acad. Nutr. Diet. 2012, 112, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Voitk, A.J.; Brown, R.A.; McArdle, A.H.; Hinchey, E.J.; Gurd, F.N. Clinical uses of an elemental diet: Preliminary studies. Can. Med. Assoc. J. 1972, 107, 123–129. [Google Scholar] [PubMed]
- Voitk, A.J.; Echave, V.; Feller, J.H.; Brown, R.A.; Gurd, F.N. Experience with elemental diet in the treatment of inflammatory bowel disease. Is this primary therapy? Arch. Surg. 1973, 107, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Palchaudhuri, S.; Albenberg, L.; Lewis, J. Diet Recommendations for Hospitalized Patients with Inflammatory Bowel Disease: Better Options than Nil Per Os. Crohn's Colitis 360 2020. [Google Scholar] [CrossRef]
- Dziechciarz, P.; Horvath, A.; Shamir, R.; Szajewska, H. Meta-analysis: Enteral nutrition in active Crohn's disease in children. Aliment. Pharmacol. Ther. 2007, 26, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.; Suskind, D.L. Exclusive enteral nutrition in pediatric inflammatory bowel disease. Curr. Opin. Pediatr. 2018, 30, 671–676. [Google Scholar] [CrossRef]
- Ruemmele, F.M.; Veres, G.; Kolho, K.L.; Griffiths, A.; Levine, A.; Escher, J.C.; Amil Dias, J.; Barabino, A.; Braegger, C.P.; Bronsky, J.; et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn's disease. J. Crohn's Colitis 2014, 8, 1179–1207. [Google Scholar] [CrossRef]
- Schwerd, T.; Frivolt, K.; Clavel, T.; Lagkouvardos, I.; Katona, G.; Mayr, D.; Uhlig, H.H.; Haller, D.; Koletzko, S.; Bufler, P. Exclusive enteral nutrition in active pediatric Crohn disease: Effects on intestinal microbiota and immune regulation. J. Allergy Clin. Immunol. 2016, 138, 592–596. [Google Scholar] [CrossRef]
- Day, A.S. The impact of exclusive enteral nutrition on the intestinal microbiota in inflammatory bowel disease. Aims Microbiol. 2018, 4, 584–593. [Google Scholar] [CrossRef]
- Yamamoto, T.; Nakahigashi, M.; Umegae, S.; Kitagawa, T.; Matsumoto, K. Impact of elemental diet on mucosal inflammation in patients with active Crohn's disease: Cytokine production and endoscopic and histological findings. Inflamm. Bowel Dis. 2005, 11, 580–588. [Google Scholar] [CrossRef]
- Rolandsdotter, H.; Jonsson-Videsater, K.; Fagerberg, U.L.; Finkel, Y.; Eberhardson, M. Exclusive Enteral Nutrition: Clinical Effects and Changes in Mucosal Cytokine Profile in Pediatric New Inflammatory Bowel Disease. Nutrients 2019, 11, 414. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, A.M.; Ohlsson, A.; Sherman, P.M.; Sutherland, L.R. Meta-analysis of enteral nutrition as a primary treatment of active Crohn's disease. Gastroenterology 1995, 108, 1056–1067. [Google Scholar] [CrossRef]
- Narula, N.; Dhillon, A.; Zhang, D.; Sherlock, M.E.; Tondeur, M.; Zachos, M. Enteral nutritional therapy for induction of remission in Crohn's disease. Cochrane Database Syst. Rev. 2018, 4, CD000542. [Google Scholar] [CrossRef] [PubMed]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68, s1–s106. [Google Scholar] [CrossRef] [PubMed]
- Heerasing, N.; Thompson, B.; Hendy, P.; Heap, G.A.; Walker, G.; Bethune, R.; Mansfield, S.; Calvert, C.; Kennedy, N.A.; Ahmad, T.; et al. Exclusive enteral nutrition provides an effective bridge to safer interval elective surgery for adults with Crohn's disease. Aliment. Pharmacol. Ther. 2017, 45, 660–669. [Google Scholar] [CrossRef]
- Takagi, S.; Utsunomiya, K.; Kuriyama, S.; Yokoyama, H.; Takahashi, S.; Iwabuchi, M.; Takahashi, H.; Takahashi, S.; Kinouchi, Y.; Hiwatashi, N.; et al. Effectiveness of an 'half elemental diet' as maintenance therapy for Crohn's disease: A randomized-controlled trial. Aliment. Pharmacol. Ther. 2006, 24, 1333–1340. [Google Scholar] [CrossRef]
- Sazuka, S.; Katsuno, T.; Nakagawa, T.; Saito, M.; Saito, K.; Matsumura, T.; Arai, M.; Sato, T.; Yokosuka, O. Concomitant use of enteral nutrition therapy is associated with sustained response to infliximab in patients with Crohn's disease. Eur. J. Clin. Nutr. 2012, 66, 1219–1223. [Google Scholar] [CrossRef]
- Hirai, F.; Ishihara, H.; Yada, S.; Esaki, M.; Ohwan, T.; Nozaki, R.; Ashizuka, S.; Inatsu, H.; Ohi, H.; Aoyagi, K.; et al. Effectiveness of concomitant enteral nutrition therapy and infliximab for maintenance treatment of Crohn's disease in adults. Dig. Dis. Sci. 2013, 58, 1329–1334. [Google Scholar] [CrossRef]
- Nguyen, D.L.; Palmer, L.B.; Nguyen, E.T.; McClave, S.A.; Martindale, R.G.; Bechtold, M.L. Specialized enteral nutrition therapy in Crohn's disease patients on maintenance infliximab therapy: A meta-analysis. Ther. Adv. Gastroenterol. 2015, 8, 168–175. [Google Scholar] [CrossRef]
- Nguyen, G.C.; Laveist, T.A.; Brant, S.R. The utilization of parenteral nutrition during the in-patient management of inflammatory bowel disease in the United States: A national survey. Aliment. Pharmacol. Ther. 2007, 26, 1499–1507. [Google Scholar] [CrossRef]
- Issa, M.; Binion, D.G. Bowel rest and nutrition therapy in the management of active Crohn's disease. Nutr. Clin. Pract. 2008, 23, 299–308. [Google Scholar] [CrossRef]
- Mihai, C.; Prelipcean, C.C.; Pintilie, I.; Nedelciuc, O.; Jigaranu, A.O.; Dranga, M.; Mihai, B. Nutrition in inflammatory bowel diseases. Rev. Med. Chir. A Soc. Med. Nat. Din Iasi 2013, 117, 662–669. [Google Scholar]
- Wedrychowicz, A.; Zajac, A.; Tomasik, P. Advances in nutritional therapy in inflammatory bowel diseases: Review. World J. Gastroenterol. 2016, 22, 1045–1066. [Google Scholar] [CrossRef]
- Makhdoom, Z.A.; Komar, M.J.; Still, C.D. Nutrition and enterocutaneous fistulas. J. Clin. Gastroenterol. 2000, 31, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Martincich, I.; Cini, K.; Lapkin, S.; Lord, H.; Fernandez, R. Central Venous Access Device Complications in Patients Receiving Parenteral Nutrition in General Ward Settings: A Retrospective Analysis. J. Parenter. Enter. Nutr. 2020, 44, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Ukleja, A.; Romano, M.M. Complications of parenteral nutrition. Gastroenterol. Clin. N. Am. 2007, 36, 23–46. [Google Scholar] [CrossRef]
- Miehsler, W.; Reinisch, W.; Valic, E.; Osterode, W.; Tillinger, W.; Feichtenschlager, T.; Grisar, J.; Machold, K.; Scholz, S.; Vogelsang, H.; et al. Is inflammatory bowel disease an independent and disease specific risk factor for thromboembolism? Gut 2004, 53, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Faye, A.S. Venous thromboembolism in inflammatory bowel disease. World J. Gastroenterol. 2020, 26, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Egberg, M.D.; Galanko, J.A.; Barnes, E.L.; Kappelman, M.D. Thrombotic and Infectious Risks of Parenteral Nutrition in Hospitalized Pediatric Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019, 25, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.C.; Rampertab, S.D.; Mullin, G.E. Existing dietary guidelines for Crohn's disease and ulcerative colitis. Expert Rev. Gastroenterol. Hepatol. 2011, 5, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, G.R.; Loftus, E.V.; Isaacs, K.L.; Regueiro, M.D.; Gerson, L.B.; Sands, B.E. ACG Clinical Guideline: Management of Crohn's Disease in Adults. Am. J. Gastroenterol. 2018, 113, 481–517. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.B.; Lee, D.; Long, M.D.; Kappelman, M.D.; Martin, C.F.; Sandler, R.S.; Lewis, J.D. Dietary patterns and self-reported associations of diet with symptoms of inflammatory bowel disease. Dig. Dis. Sci. 2013, 58, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Frolkis, A.D.; Dykeman, J.; Negron, M.E.; Debruyn, J.; Jette, N.; Fiest, K.M.; Frolkis, T.; Barkema, H.W.; Rioux, K.P.; Panaccione, R.; et al. Risk of surgery for inflammatory bowel diseases has decreased over time: A systematic review and meta-analysis of population-based studies. Gastroenterology 2013, 145, 996–1006. [Google Scholar] [CrossRef]
- Bouguen, G.; Peyrin-Biroulet, L. Surgery for adult Crohn's disease: What is the actual risk? Gut 2011, 60, 1178–1181. [Google Scholar] [CrossRef]
- Patel, K.V.; Darakhshan, A.A.; Griffin, N.; Williams, A.B.; Sanderson, J.D.; Irving, P.M. Patient optimization for surgery relating to Crohn's disease. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 707–719. [Google Scholar] [CrossRef]
- Studley, H.O. Percentage of weight loss: A basic indicator of surgical risk in patients with chronic peptic ulcer. 1936. Nutr. Hosp. 2001, 16, 141–143. [Google Scholar]
- Adamina, M.; Gerasimidis, K.; Sigall-Boneh, R.; Zmora, O.; de Buck van Overstraeten, A.; Campmans-Kuijpers, M.; Ellul, P.; Katsanos, K.; Kotze, P.G.; Noor, N.; et al. Perioperative Dietary Therapy in Inflammatory Bowel Disease. J. Crohn's Colitis 2020, 14, 431–444. [Google Scholar] [CrossRef]
- Kondrup, J.; Rasmussen, H.H.; Hamberg, O.; Stanga, Z.; Ad Hoc, E.W.G. Nutritional risk screening (NRS 2002): A new method based on an analysis of controlled clinical trials. Clin. Nutr. 2003, 22, 321–336. [Google Scholar] [CrossRef]
- Sandhu, A.; Mosli, M.; Yan, B.; Wu, T.; Gregor, J.; Chande, N.; Ponich, T.; Beaton, M.; Rahman, A. Self-Screening for Malnutrition Risk in Outpatient Inflammatory Bowel Disease Patients Using the Malnutrition Universal Screening Tool (MUST). J. Parenter. Enter. Nutr. 2016, 40, 507–510. [Google Scholar] [CrossRef]
- Schiesser, M.; Muller, S.; Kirchhoff, P.; Breitenstein, S.; Schafer, M.; Clavien, P.A. Assessment of a novel screening score for nutritional risk in predicting complications in gastro-intestinal surgery. Clin. Nutr. 2008, 27, 565–570. [Google Scholar] [CrossRef]
- Adamina, M.; Bonovas, S.; Raine, T.; Spinelli, A.; Warusavitarne, J.; Armuzzi, A.; Bachmann, O.; Bager, P.; Biancone, L.; Bokemeyer, B.; et al. ECCO Guidelines on Therapeutics in Crohn's Disease: Surgical Treatment. J. Crohn's Colitis 2020, 14, 155–168. [Google Scholar] [CrossRef]
- Zerbib, P.; Koriche, D.; Truant, S.; Bouras, A.F.; Vernier-Massouille, G.; Seguy, D.; Pruvot, F.R.; Cortot, A.; Colombel, J.F. Pre-operative management is associated with low rate of post-operative morbidity in penetrating Crohn's disease. Aliment. Pharmacol. Ther. 2010, 32, 459–465. [Google Scholar] [CrossRef]
- Marimuthu, K.; Varadhan, K.K.; Ljungqvist, O.; Lobo, D.N. A meta-analysis of the effect of combinations of immune modulating nutrients on outcome in patients undergoing major open gastrointestinal surgery. Ann. Surg. 2012, 255, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Francis, N.K.; Walker, T.; Carter, F.; Hubner, M.; Balfour, A.; Jakobsen, D.H.; Burch, J.; Wasylak, T.; Demartines, N.; Lobo, D.N.; et al. Consensus on Training and Implementation of Enhanced Recovery after Surgery: A Delphi Study. World J. Surg. 2018, 42, 1919–1928. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, S. Early postoperative complications in patients with Crohn's disease given and not given preoperative total parenteral nutrition. Scand. J. Gastroenterol. 2012, 47, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Lashner, B.A.; Evans, A.A.; Hanauer, S.B. Preoperative total parenteral nutrition for bowel resection in Crohn's disease. Dig. Dis. Sci. 1989, 34, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Grivceva Stardelova, K.; Misevska, P.; Zdravkovska, M.; Trajkov, D.; Serafimoski, V. Total parenteral nutrition in treatment of patients with inflammatory bowel disease. Prilozi 2008, 29, 21–43. [Google Scholar]
- Bellolio, F.; Cohen, Z.; Macrae, H.M.; O'Connor, B.I.; Huang, H.; Victor, J.C.; McLeod, R.S. Outcomes following surgery for perforating Crohn's disease. Br. J. Surg. 2013, 100, 1344–1348. [Google Scholar] [CrossRef]
- Brennan, G.T.; Ha, I.; Hogan, C.; Nguyen, E.; Jamal, M.M.; Bechtold, M.L.; Nguyen, D.L. Does preoperative enteral or parenteral nutrition reduce postoperative complications in Crohn's disease patients: A meta-analysis. Eur. J. Gastroenterol. Hepatol. 2018, 30, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Ren, J.; Wang, G.; Hu, D.; Gu, G.; Liu, S.; Ren, H.; Wu, X.; Li, J. Preoperative exclusive enteral nutrition reduces the postoperative septic complications of fistulizing Crohn's disease. Eur. J. Clin. Nutr. 2014, 68, 441–446. [Google Scholar] [CrossRef]
- Li, Y.; Zuo, L.; Zhu, W.; Gong, J.; Zhang, W.; Gu, L.; Guo, Z.; Cao, L.; Li, N.; Li, J. Role of exclusive enteral nutrition in the preoperative optimization of patients with Crohn's disease following immunosuppressive therapy. Medicine 2015, 94, e478. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zuo, L.; Zhao, J.; Dong, J.; Li, Y.; Gu, L.; Gong, J.; Liu, Q.; Zhu, W. Impact of Preoperative Exclusive Enteral Nutrition on Postoperative Complications and Recurrence after Bowel Resection in Patients with Active Crohn's Disease. World J. Surg. 2016, 40, 1993–2000. [Google Scholar] [CrossRef] [PubMed]
- Smedh, K.; Andersson, M.; Johansson, H.; Hagberg, T. Preoperative management is more important than choice of sutured or stapled anastomosis in Crohn's disease. Eur. J. Surg. Acta Chir. 2002, 168, 154–157. [Google Scholar] [CrossRef]
- Ianco, O.; Tulchinsky, H.; Lusthaus, M.; Ofer, A.; Santo, E.; Vaisman, N.; Dotan, I. Diet of patients after pouch surgery may affect pouch inflammation. World J. Gastroenterol. 2013, 19, 6458–6464. [Google Scholar] [CrossRef]
- Godny, L.; Maharshak, N.; Reshef, L.; Goren, I.; Yahav, L.; Fliss-Isakov, N.; Gophna, U.; Tulchinsky, H.; Dotan, I. Fruit Consumption is Associated with Alterations in Microbial Composition and Lower Rates of Pouchitis. J. Crohn's Colitis 2019, 13, 1265–1272. [Google Scholar] [CrossRef]
- Godny, L.; Reshef, L.; Pfeffer-Gik, T.; Goren, I.; Yanai, H.; Tulchinsky, H.; Gophna, U.; Dotan, I. Adherence to the Mediterranean diet is associated with decreased fecal calprotectin in patients with ulcerative colitis after pouch surgery. Eur. J. Nutr. 2020, 59, 3183–3190. [Google Scholar] [CrossRef] [PubMed]
Figure 1. Pro-inflammatory and anti-inflammatory nutrients influence the risk of inflammatory bowel disease (IBD). Micronutrient deficiency is an important consequence of inflammatory bowel disease. Dietetic interventions, in particular exclusive enteral nutrition in children with Crohn's disease, are effective in inducing remission.
Figure 1. Pro-inflammatory and anti-inflammatory nutrients influence the risk of inflammatory bowel disease (IBD). Micronutrient deficiency is an important consequence of inflammatory bowel disease. Dietetic interventions, in particular exclusive enteral nutrition in children with Crohn's disease, are effective in inducing remission.
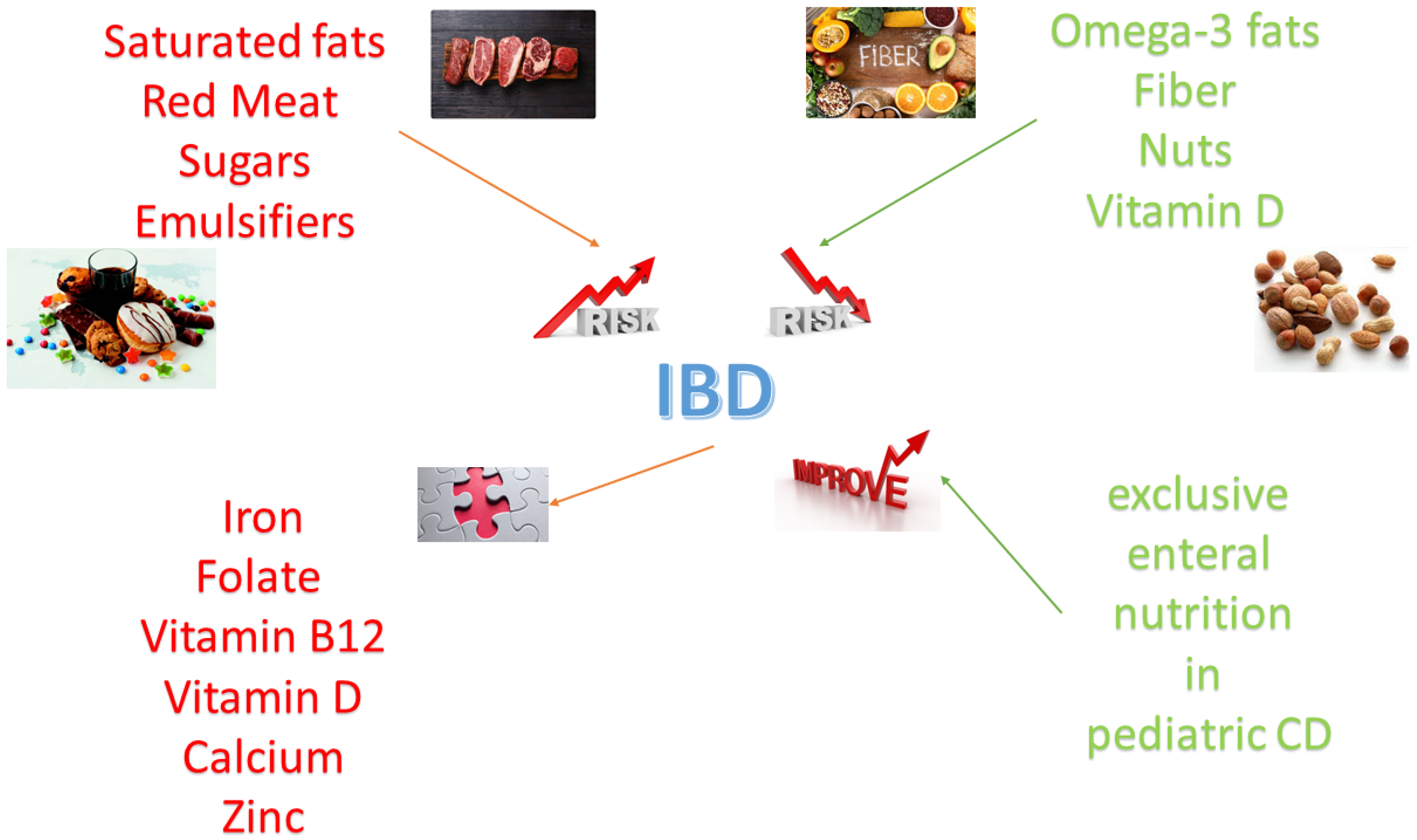
Figure 2. Western diet is a risk fact for inflammatory bowel disease because it causes intestinal dysbiosis and inflammation; one important consequence of inflammatory bowel disease is malnutrition. Micronutrient supplementation and dietary intervention could counterbalance these effects. IBD: Inflammatory bowel disease; EEN: Exclusive enteral nutrition.
Figure 2. Western diet is a risk fact for inflammatory bowel disease because it causes intestinal dysbiosis and inflammation; one important consequence of inflammatory bowel disease is malnutrition. Micronutrient supplementation and dietary intervention could counterbalance these effects. IBD: Inflammatory bowel disease; EEN: Exclusive enteral nutrition.
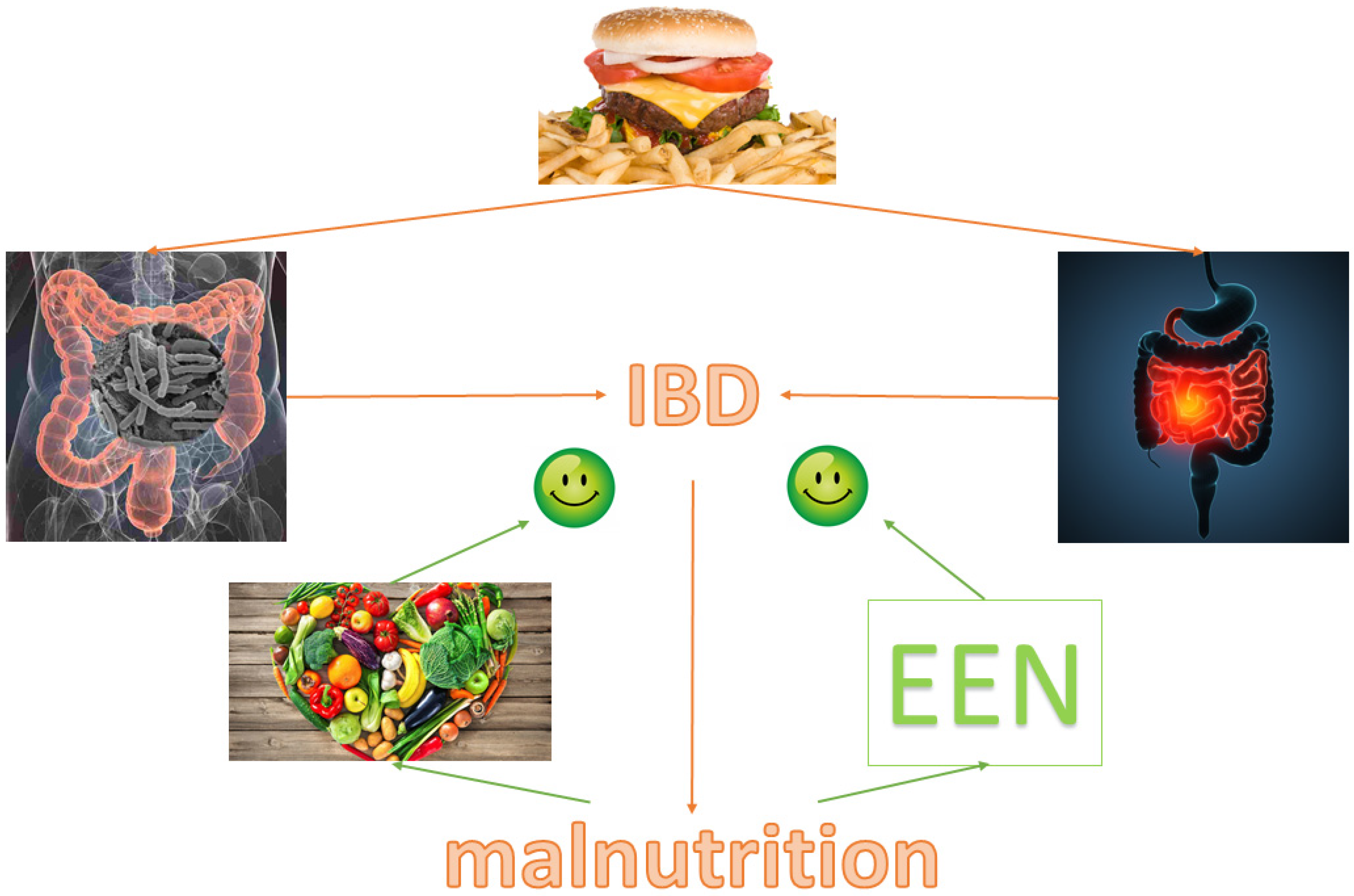
Table 1. Micronutrients deficiency in patients affected by IBD.
Table 1. Micronutrients deficiency in patients affected by IBD.
| Micronutrient | Population Target | Intervals | Supplementation | Consequences |
|---|---|---|---|---|
| Iron [64] | All patients | - Annually - if evidence of deficiency: Every 3 months for the first year, 6–12 months thereafter | - Mild or moderate anemia: - New oral formulation like ferrous bysglicinate chelate - Severe anemia or intolerant patients: - New intravenous formulation like ferric carboxymaltose | Anemia; Fatigue; Hair loss |
| Folate [65] | All patients | - Annually - if evidence of deficiency: Every 3 months for the first year, 6–12 months thereafter | 5 mg of folate per day | Anemia; Fatigue; Hyperhomocysteinemia |
| Vitamin B12 [66] | All patients | - Annually - if evidence of deficiency: Every 3 months for the first year, 6–12 months thereafter | 5000 microg intramuscularly or sublingual every month | Anemia; Fatigue; Hyperhomocysteinemia; Peripheral neuropathy |
| Vitamin D [67] | All patients | - Annually - if evidence of deficiency: Every 3 months for the first year, 6–12 months thereafter | - 25.000 to 50.000 UI of cholecalciferol per month orally - In case of severe malabsorption, 300.000 UI intramuscularly every six months | Osteoporosis; Worse disease course |
| Calcium [68] | Intestinal failure | - According to the deficiency | - Orally up to 3000 mg per day, plus intravenously according to the deficiency | Tetany; Osteoporosis |
| Zinc [69] | Intestinal failure | - According to the deficiency | - 40–110 mg three times a day for 8 weeks orally | Dysgeusia; Poor wound healing; Dermatitis; Failure to thrive |
Table 2. The main studies evaluating nutritional support in surgical patients with IBD.
Table 2. The main studies evaluating nutritional support in surgical patients with IBD.
| Nutritional Route | Type of Study | Main Findings | |
|---|---|---|---|
| Jacobson [167] | TPN | Case series (TPN vs. no nutritional support) | No complications in patients treated with TPN |
| Lashner et al. [168] | TPN | Retrospective study (TPN vs. no nutritional support) | Longer hospital stay in TPN; shorter resection length in TPN |
| Grivceva Stardelova et al. [169] | TPN | Retrospective study (TPN vs. no nutritional support) | Higher increase in BMI in TPN |
| Bellolio et al. [170] | TPN | Retrospective study (perforating vs. non-perforating disease) | TPN was associated with perforating disease |
| Brennan et al. [171] | TPN EEN | Meta-analysis TPN vs. EEN | EEN reduced postoperative morbidity |
| Zerbib et al. [164] | TPN EEN | Retrospective study evaluating complications in surgical patients | EEN reduced more than TPN septic complications |
| Li et al. [172] | EEN | Retrospective study EEN vs. no nutritional support | EEN reduced septic complications |
| Li et al. [173] | EEN | Retrospective study EEN vs. no nutritional support | EEN was associated with lower rate of stoma creation and with low postoperative complications |
| Wang et al. [174] | EEN | Prospective study EEN vs. no nutritional support | EEN increased nutritional parameters and was associated with low complications |
| Smedh et al. [175] | EEN | Prospective study on surgical techniques | Patients treated with EEN showed lower surgical complications |
| Publisher's Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Source: https://www.mdpi.com/2072-6643/13/4/1387/htm
0 Response to "Dr Fusco's High Fiber Diet"
Post a Comment